The post 2016 Year in Review: An Opportunity to Reflect on Highlights and Groundbreaking Accomplishments appeared first on Adaptable.
]]>happy, healthy, and safe holiday and all the best in the New Year. This year has been a memorable year for ADAPTABLE and we would like to take a moment to reflect on accomplishments during 2016, several of which were groundbreaking and could not have been achieved without the hard work and dedication of our patient partners, clinicians, researchers, and study leaders.

One of the highlights for many ADAPTABLE team members was the opportunity to meet in person and to interact with our patient partners at the ADAPTABLE Post Enrollment Kick-off Meeting. Watch this video for an inside glimpse of the meeting.
We hope that you join us in celebrating the many successes of 2016:
- Launched ADAPTABLE Portal providing e-consent and direct patient follow-up. Patient partners played a key role in development of the consent form and portal design.
- Enrolled the first participant in April at Vanderbilt. All eight participating Clinical Data Research Networks (CDRNs) have now activated at least 1 site.
- Identified over 190,000 eligible patients in participating healthcare systems using an electronic computable phenotype.
- Launched the patient partnership with our ADAPTOR team and Health eHeart Alliance, a cardiovascular Patient Powered Research Network, in all aspects of the trial including key insights into development of the consent form, trial portal, and recruitment strategies.
- Successful Post-Enrollment Kick-off Meeting in October bringing clinicians, researchers, and patient partners together. Establishing four working groups based on key discussions that will be launched in early 2017.
- Endorsement by the American College of Cardiology (ACC) Board of Governors and dissemination of ADAPTABLE information to all ACC state chapters.
- Support from the American Heart Association (AHA) for the goals and objectives of the trial.
- Established partnership with ACC and AHA patient engagement teams for collaborative outreach campaigns utilizing social media channels.
- Held inaugural Facebook Live event at AHA in partnership with REACHnet that had over 6,000 views and reached over 30,000 people.
- Approached more than 20,000 patients for recruitment, many through utilizing large scale approach methods.
- Enrolled almost 700 patients at 15 sites, over 8 months in the United States – well above any prior experiences with traditional trials in that time span.
- Distributed the first prep-to-research query for ADAPTABLE accessing overall readiness of datamarts. First ADAPTABLE specific query will be sent in 2017.
- Developed, finalized, and distributed a protocol amendment with input from the various ADAPTABLE committees, CDRNs, and ADAPTORS.
The post 2016 Year in Review: An Opportunity to Reflect on Highlights and Groundbreaking Accomplishments appeared first on Adaptable.
]]>The post Facebook Live Broadcast: What is the Optimal Dose of Aspirin for Patients with Heart Disease? appeared first on Adaptable.
]]>
The ADAPTABLE study is being presented to fellow clinicians, researchers and scientists attending Scientific Sessions, Nov. 12th – Nov 16th in New Orleans. #AHA16 is the leading cardiovascular meeting in the country with more than 17,000 professionals attending annually. The scientific programming is designed to improve patient care by providing a forum for researchers and scientists to share significant advances in prevention, diagnosis and treatment of cardiovascular disease from different perspectives. www.scientificsessions.org
Join us on November 15 at 10:15 CST for the LIVE Q&A by visiting: www.facebook.com/events/363697957306812/
The post Facebook Live Broadcast: What is the Optimal Dose of Aspirin for Patients with Heart Disease? appeared first on Adaptable.
]]>Continue Reading
The post ADAPTABLE Study Kick-Off Meeting: Determining the Path Forward for ADAPTABLE and Pragmatic Research appeared first on Adaptable.
]]>
The meeting kicked off with welcome remarks from the Co-Principal Investigators, Matthew Roe, MD, MHS and Adrian Hernandez, MD, MHS from the Duke Clinical Research Institute Coordinating Center. In opening comments, Joe Selby, MD, MPH, Executive Director of the Patient-Centered Outcomes Research Institute (PCORI) and Russell Rothman, MD, MPP, ADAPTABLE Study Co-Chair, provided the framework for how the ADAPTABLE study is breaking ground in the conduct of pragmatic trials. Selby discussed how studies like ADAPTABLE are demonstrating the power of PCORnet, the National Patient-Centered Clinical Research Network, whose mission is to “ enable people to make informed healthcare decisions by efficiently conducting clinical research relevant to their needs.” “You are laying down the tracks to pragmatic research while driving ADAPTABLE forward as a model of faster, less expensive, and people-centered approach to clinical research,” said Selby. Rothman added, “The ADAPTABLE study represents an innovative opportunity to revolutionize clinical research by leveraging health information technology that can lead to rapid participant identification, contact, consent, and data collection. In addition, the engagement of patients, clinicians and other key stakeholders in all aspects of ADAPTABLE from design through dissemination will allow us to do research that is improving healthcare and is of greatest value to the community.”
Rachael Fleurence, PhD, PCORI, Program Director and Chair of the PCORnet Executive Committee congratulated the Clinical Data Research Networks (CDRNs) who are currently enrolling participants and acknowledged that the remaining networks are on the cusp of enrolling. “When you are breaking new ground, it can take time to overcome challenges and see progress, but I can tell ADAPTABLE is picking up steam,” said Fleurence.
Moving the Needle from Traditional to Pragmatic Research
Robert Harrington, MD, ADAPTABLE Study Co-Chair described how pragmatic trials are evolving from the model of traditional trials. Traditional trials are expensive, long, and often don’t provide critical evidence. With costs typically at least hundreds of millions of dollars and taking more than five years to obtain results, the traditional model of research is not sustainable. In addition, these trials often do not answer questions people have about their health care. Harrington and meeting attendees agreed that while results from traditional trials are published in scientific journals and presented at professional meetings, the participants ─ who agreed to be in the study, underwent the protocol procedures, and provided their data ─ often never learn about study results.
Enter ADAPTABLE, a new model of pragmatic research with a focus on transparency and engagement. ADAPTABLE will compare the effectiveness of two different daily doses (81 mg and 325 mg) of aspirin widely used to prevent heart attacks and strokes in individuals living with heart disease. Using electronic health records (EHRs) to screen and identify potential participants against key indicators, efficiencies gained in ADAPTABLE include: 1) Elimination of data-entry redundancies by obtaining information directly from EHRs and data in the PCORnet Common Data Model; 2) Reporting outcomes data by participants directly via the ADAPTABLE web portal; 3) Elimination of monitoring costs.
How do we answer the age-old aspirin question?
Adrian Hernandez, MD, MHS, ADAPTABLE Study Co-PI and PCORnet Co-PI started the operations update presentation with a question he hears from his mother who has cardiovascular disease, “How do you not know the right dose of aspirin?” It was no surprise to the meeting attendees that although doctors prescribe either low-dose aspirin (81 mg) or regular-strength aspirin (325mg daily) to help prevent a future heart attack, stroke, or death, there is no clear, long-term evidence to delineate the best dose of aspirin to prescribe for patients with cardiovascular disease.
“It would be too expensive and take too long for a company from the pharmaceutical industry to answer this aspirin dose question. However, it is a perfect question to show the power of PCORnet to harness massive amounts of health data to answer questions such as this one and other that matter to people,” said Hernandez. Read more about PCORnet here.
Success in the Making
ADAPTABLE Study Co-PI, Matthew Roe, MD, MHS reviewed metrics highlighting early successes and challenges. While ADAPTABLE start up timelines are slightly longer for Institutional Review Board (IRB) approval and site activation compared with traditional cardiovascular outcomes trials, the average monthly enrollment rate per site is significantly higher than the average monthly enrollment rate for a site in a traditional trial. IRBs often have little experience with pragmatic trials so longer trial reviews are not unusual.
Each CDRN presented their site-level metrics, recruitment approaches, and enrollment plans. These presentations were followed by an engaging group discussion of lessons learned. The attendees discussed topics such as: How pragmatic do we need to be when facing an enrollment goal of 20,000? The group agreed that pragmatism is a spectrum and that each CDRN will need to evaluate recruitment approaches and find what fits best at their site.
The group overwhelmingly agreed that a broad, multi-touch approach is needed for the outreach to potential participants to achieve the trial enrollment goals. This type of approach includes contacting eligible patients in a variety of ways, including by email, phone, direct mail, and facilitated discussions during clinic encounters with tablets and other mechanisms. Participants also stressed the importance of clinician engagement. “Primary care providers and cardiologists can support their patients and ADAPTABLE by having discussions with their colleagues about ADAPTABLE and why it is important to have broad support,” said Roe. Just as in traditional trials, patients like to hear about studies from their physician and know that their physician is in support of their participation. The challenge will be how to optimize that message with the electronic recruitment approaches.
Real People Shaping Research

Without question having the Adaptors ─ the ADAPTABLE patient leaders and partners ─ engaged in the meeting discussions and during panel discussions was the highlight of the meeting for all attendees. The Adaptors are patient investigators from each of the eight participating CDRNs who have already provided protocol feedback, review of the consent form, and input on the study portal and materials. The Health eHeart Alliance PPRN supports and facilitates the Adaptors Team via bi-weekly check-in calls.
This meeting provided a face-to-face opportunity for researchers and Adaptors to interact and all agreed that it was an amazing experience. Seven of the eight ADAPTORS were able to attend the kick-off meeting and participated in a facilitated panel discussion. “Prior to my cardiac event, I had lost three close friends to heart disease,” shared Ken Gregoire from the REACHnet CDRN. “When I was introduced to ADAPTABLE by Oschner, I thought the results from this study might benefit someone I know or even someone I don’t know. I wanted to be involved to get the word out about ADAPTABLE.”
By being present at the meeting, the Adaptors heard of the recruitment challenges and learned of opportunities where they could be involved as study ambassadors by making other patients aware of ADAPTABLE. Linda Brown from the Mid-South CDRN, whose father and brothers all died from heart disease before they were 65 years old, is grateful to be involved to help support her husband and other patients with heart disease. “I think the Adaptors can play a pivotal role in publically sharing study updates and results in lay terms so that other patients will have a better understanding of what the results mean.” Brown said. “The act of sharing study progress may encourage more people to enroll in studies knowing that they will be informed of the results and how their participation contributed.”
Adaptors Team Co-Chairs, Jacqueline Alikhaani from pSCANNER CDRN and Henry Cruz from NYC-CDRN echoed the importance of including the patient voice in clinical research. “We cannot improve patient care without involving patients in the process,” said Alikhaaini. “In ADAPTABLE, we have the opportunity to develop innovative ways to engage the patient community.” Cruz, who is an advocate for diabetes education after his mother and nine siblings died from complications of diabetes said, “Providing the patient perspective to health research is my passion. Telling my personal story makes me vulnerable but I know in my heart that clinical research has the potential to save lives.”
The Path Forward
As with all clinical trials, traditional or pragmatic, opportunities to establish and deepen personal relationships can play a significant role in the success of a study. The probing questions, thought-provoking presentations and high-level dialogue at the kick-off meeting help to further develop the personal relationships that are critical for the success of clinical trials, especially a novel, pragmatic trial like ADAPTABLE. Continue to follow the success of ADAPTABLE by visiting the website and following on Twitter @ADAPTABLEstudy.
The ADAPTABLE study is funded through a Patient-Centered Outcomes Research Institute (PCORI) Award (Contract Number: ASP-1502-27079).ADAPTABLE is the first demonstration project to be conducted through PCORnet, the National Patient-Centered Clinical Research Network. PCORnet is a PCORI-funded initiative designed to improve the nation’s capacity to conduct comparative clinical effectiveness research. More information about PCORnet can be found here.
The post ADAPTABLE Study Kick-Off Meeting: Determining the Path Forward for ADAPTABLE and Pragmatic Research appeared first on Adaptable.
]]>Continue Reading
The post ADAPTABLE Study Colleagues Showcase its Unprecedented Patient-Centricity at AHA Conference appeared first on Adaptable.
]]>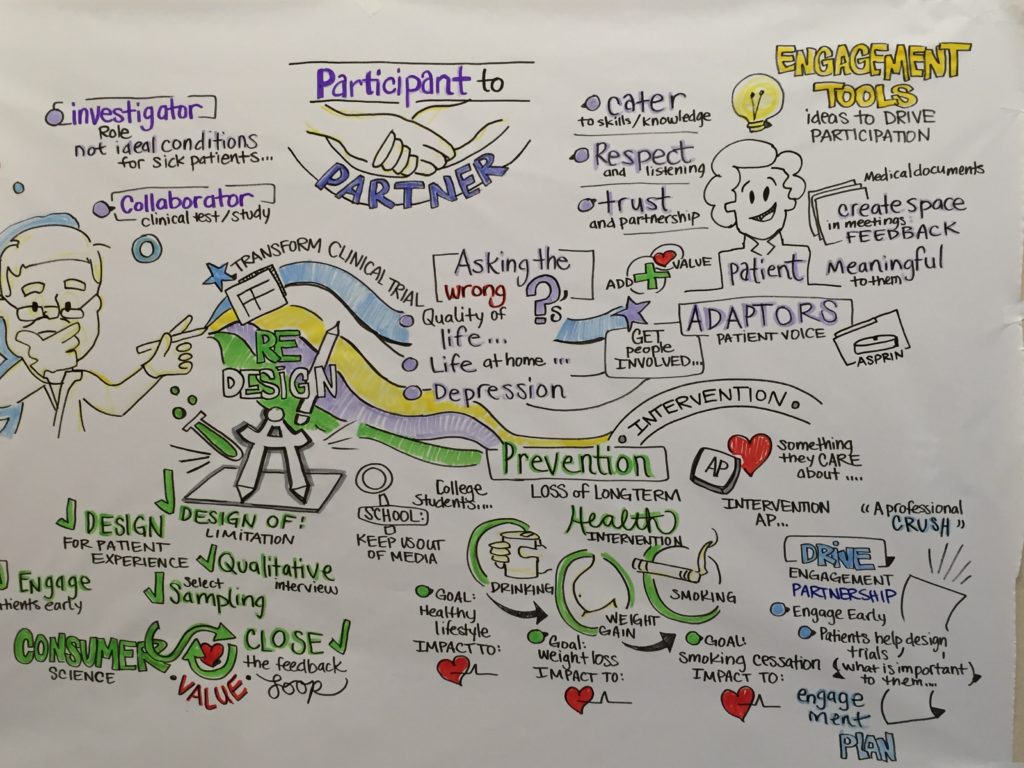
Dr. Adrian Hernandez, director of Health Services and Outcomes Research at Duke Clinical Research Institute (DCRI) and Madelaine Faulkner, Health-eHeart Alliance Project director, recently hosted a sunrise session on the value of patient-centricity in clinical research at the inaugural American Heart Association (AHA) Research Academy conference. Using ADAPTABLE (Aspirin Dosing: A Patient-centric Trial Assessing Benefits and Long-Term Effectiveness) as an illustration, Hernandez and Faulkner offered the conference’s nearly 300 attendees tips for breaking down the language barrier that often exists between patients and physicians and giving patients a voice in the clinical research process.
“The inaugural AHA Research Academy conference was a fantastic opportunity to network and share insights with some of the most prominent thought leaders in cardio- and cerebrovascular disease,” said Hernandez. “Our work to drive patient-centricity with ADAPTABLE was well received by the conference attendees, which underscores our goal at PCORnet, the National Patient-Centered Clinical Research Network, to transform the culture of clinical research from one directed by researchers to one driven by the needs of patients and those who care for them.”
ADAPTABLE is funded through a research award totaling up to $18 million from the Patient-Centered Outcomes Research Institute (PCORI). DCRI is the Coordinating Center for this first clinical research study of PCORnet, an innovative initiative of PCORI involving 33 individual networks working together to make it faster, easier, and less costly to conduct clinical research by harnessing the power of large amounts of health data and patient partnerships. The study plans to enroll 20,000 patients with heart disease identified from electronic health records from eight of PCORnet’s Clinical Data Research Networks (CDRNs) and 30 different health systems. The Health eHeart Alliance Patient-Powered Research Network (PPRN) supports the Adaptors, a team of people with coronary heart disease who helped design the protocol, consent form, portal content, and study materials. Together the Adaptors and Health eHeart Alliance have developed patient-centered processes to facilitate engagement and provide the patient perspective that is so often missing in the clinical research process.
While Hernandez opened the session with an overview of patient centricity’s value, it was Faulkner’s presentation that examined the nuts and bolts of how ADAPTABLE is bringing patient-centered research to life.
“The input from Adaptors helps us to break down the language barrier that often exists between patients and physicians,” said Faulkner. “We get feedback that we cannot speak in acronyms all the time and that we need to make research approachable to communicate its value. When we break down these communication barriers, we deepen our engagement with our patient leaders — and hopefully this will transfer to the broader patient population as well.”
In addition to the perspectives brought by Hernandez and Faulkner, Jamie Roberts of the Clinical Trials Transformation Initiative (CTTI), a Duke/FDA public-private partnership, described CTTI’s published Best Practices for Patient Group Engagement and how the CTTI project team is now working to understand and demonstrate the value of patient group engagement to industry and academic research sponsors.
Overall, the AHA Research Academy was a positive and insightful meeting that provided not only a venue to showcase ADAPTABLE’s innovative patient-centric approach, but also a learning opportunity to glean insights from the academic community.
“I saw a number of thought-provoking presentations, including one with an excellent quote by an information technology health strategist named Leonard Kish,” said Hernandez. “Kish said, ‘If patient engagement were a drug, it would be the blockbuster drug of the century and malpractice not to use it.’ I wholeheartedly agree and am proud that PCORnet is pioneering patient-centric research with the ADAPTABLE study.”
###
The ADAPTABLE study is funded through a Patient-Centered Outcomes Research Institute (PCORI) Award (Contract Number: ASP-1502-27079). ADAPTABLE is the first demonstration project to be conducted through PCORnet, the National Patient-Centered Clinical Research Network. PCORnet is a PCORI-funded initiative designed to improve the nation’s capacity to conduct comparative clinical effectiveness research.
About PCORnet
PCORnet, the National Patient-Centered Clinical Research Network, is an innovative initiative of the Patient-Centered Outcomes Research Institute (PCORI). The goal of PCORnet is to improve the nation’s capacity to conduct comparative clinical effectiveness research efficiently by creating a large, highly representative network for conducting clinical outcomes research that directly involves patients in the development and execution of the research. More information is available at www.pcornet.org.
About PCORI
The Patient-Centered Outcomes Research Institute (PCORI) is an independent, nonprofit organization authorized by Congress in 2010. Its mission is to fund research that will provide patients, their caregivers, and clinicians with the evidence-based information needed to make better-informed healthcare decisions. PCORI is committed to continually seeking input from a broad range of stakeholders to guide its work. More information is available at www.pcori.org.
The post ADAPTABLE Study Colleagues Showcase its Unprecedented Patient-Centricity at AHA Conference appeared first on Adaptable.
]]>Continue Reading
The post OneFlorida Clinical Data Research Network Joins the ADAPTABLE Study appeared first on Adaptable.
]]>
ADAPTABLE is designed to compare the effectiveness of two daily doses of aspirin widely used to prevent heart attacks and strokes in individuals living with heart disease. The study hopes to generate results that will improve care and outcomes for patients with heart disease. Using electronic health records (EHRs) and web-based technology, participants will self-consent, self-randomize and report data directly through an online portal. In addition, with participants’ consent, electronic health information captured during routine care will be used to identify events that will provide ADAPTABLE researchers with additional health outcomes data to inform better decision-making.
The ADAPTABLE study is funded through a Patient-Centered Outcomes Research Institute (PCORI) Award (Contract Number: ASP-1502-27079). ADAPTABLE is the first demonstration project to be conducted through PCORnet, the National Patient-Centered Clinical Research Network. PCORnet is a PCORI-funded initiative designed to improve the nation’s capacity to conduct comparative clinical effectiveness research. More information about PCORnet can be found here.
The post OneFlorida Clinical Data Research Network Joins the ADAPTABLE Study appeared first on Adaptable.
]]>Continue Reading
The post Patients Vital to the Design of ADAPTABLE appeared first on Adaptable.
]]>In addition to answering a question of clinical importance, ADAPTABLE is unique in that its study design incorporated input from patients, physicians, and other stakeholders.
“Patients were vital to the protocol design process,” says Hernandez. “They helped design the interface for the patient portal, and they were instrumental in helping to create the patient consent form, which ensured it was patient-friendly. They are also helping us to spread the word about why it is so important to participate in this study. That is a level of patient interaction that you don’t generally see in a clinical trial.”
Hernandez is hopeful the ADAPTABLE study will change the way the industry looks at patient recruitment. Patients with heart disease will be identified electronically via inclusion/exclusion criteria based on a phenotype. Patients identified as suitable for the trial are then contacted and can learn about the trial via a web portal. After answering a few comprehension questions, they can enroll and be randomized to receive one of two doses of aspirin. Follow-up is also performed electronically via a patient portal as well as through data from the participating healthcare systems.
Click here to access the complete interview in the Clinical Leader.
The ADAPTABLE study is funded through a Patient-Centered Outcomes Research Institute (PCORI) Award (Contract Number: ASP-1502-27079). ADAPTABLE is the first demonstration project to be conducted through PCORnet, the National Patient-Centered Clinical Research Network. PCORnet is a PCORI-funded initiative designed to improve the nation’s capacity to conduct comparative clinical effectiveness research. More information about PCORnet can be found here.
The post Patients Vital to the Design of ADAPTABLE appeared first on Adaptable.
]]>Adrian F. Hernandez, MD, MHS, Director of Health Services and Outcomes Research, and Lesley Curtis, PhD, Director of the Center for Pragmatic Health Systems Research, spoke during separate sessions about their progress with the ADAPTABLE (Aspirin Dosing: A Patient-centric Trial Assessing Benefits and Long-Term Effectiveness) study.
Continue Reading
The post Researchers Discuss How ADAPTABLE Can Transform the World of Clinical Trials appeared first on Adaptable.
]]>Adrian F. Hernandez, MD, MHS, Director of Health Services and Outcomes Research, and Lesley Curtis, PhD, Director of the Center for Pragmatic Health Systems Research, spoke during separate sessions about their progress with the ADAPTABLE (Aspirin Dosing: A Patient-centric Trial Assessing Benefits and Long-Term Effectiveness) study.
Heart disease is the leading cause of death for both men and women. About 610,000 people die of heart disease in the United States every year. It accounts for one in every four deaths and is a disease that places enormous burdens not only on patients and their families but on the nation and its healthcare system as a whole. ADAPTABLE is designed to compare the effectiveness of two daily doses of aspirin widely used to prevent heart attacks and strokes in individuals living with heart disease. The study hopes to improve care and outcomes for patients with heart disease. The trial is the first pragmatic trial, or a trial designed to reflect “real-world” conditions, conducted through PCORnet, the National Patient-Centered Clinical Research Network. Uniquely, its study design incorporated input from patients, physicians, and other stakeholders.
ADAPTABLE will enroll 20,000 patients across the United States, provides a new model of enabling pragmatic research through electronic screening, consent, and follow-up. Hernandez’s session focused on the lessons learned from establishing a technological process suited for tens of thousands of patients who are being studied in a variety of clinical settings across the nation.
Using electronic health records (EHRs) and web-based technology, participants will self-consent, self-randomize and report data directly through an online portal. In addition, with participants’ consent, electronic health information captured during routine care will be used to identify events that will provide ADAPTABLE researchers with additional health outcomes data to inform better decision-making.
Curtis’ session, chaired by Duke Clinical Translational Research Institute Project Leader Shelley Rusincovitch, was devoted to a discussion of the PCORnet Common Data Model (CDM). The CDM, a key component of ADAPTABLE, is a way of organizing data into a standard structure and provides a streamlined, efficient way to use the data.
The PCORnet CDM was influenced by similar models used by initiatives such as the U.S. Food and Drug Administration (FDA) Sentinel, a program sponsored by the FDA to create an active surveillance system to monitor the safety of FDA-regulated medical products. Both Sentinel and PCORnet use a distributed data approach in which project partners maintain physical and operational control over electronic data while the CDM is used to standardize administrative and clinical information across all of the partners.
The PCORnet CDM has further expanded the possibilities for data sharing and management, Rusincovitch said.
“The CDM is powerful because of the role it plays in EHR-driven research,” she said. “If we’re working with multiple institutions and databases, the CDM provides a common foundation to build upon. That’s what makes it so useful in this context.”
The ADAPTABLE study is funded through a Patient-Centered Outcomes Research Institute (PCORI) Award (Contract Number: ASP-1502-27079). ADAPTABLE is the first demonstration project to be conducted through PCORnet, the National Patient-Centered Clinical Research Network. PCORnet is a PCORI-funded initiative designed to improve the nation’s capacity to conduct comparative clinical effectiveness research. More information about PCORnet can be found here.
The post Researchers Discuss How ADAPTABLE Can Transform the World of Clinical Trials appeared first on Adaptable.
]]>Continue Reading
The post ADAPTABLE: Answering an Important Clinical Question while Testing a New Approach to Conducting Pragmatic Clinical Trials appeared first on Adaptable.
]]>In this feature of Ask ADAPTABLE, Beth Nauman, Research Director at REACHnet, shares the network’s role in the National Patient-Centered Clinical Research Network (PCORnet) and ADAPTABLE.

What inspired REACHnet to join PCORnet?
At the Louisiana Public Health Institute, we had already established community partnerships that we engaged in our public health programming and research, so when we heard about PCORnet, we were excited about the opportunity to work with partners throughout the country on a national-level, innovative health research initiative. Our mission aligns well with PCORnet in that the primary objective of REACHnet is to make research more efficient, more informative, and less costly by harnessing the power of large amounts of health data and patient partnerships.
We began with an initial 18-month project period building health system partnerships, information technology solutions, network governance, and streamlined regulatory processes (e.g., human subjects’ protection) to establish a functioning clinical data research network (CDRN). The network was first called Louisiana Clinical Data Research Network (LaCDRN), and then we expanded into Texas and changed our name to Research Action for Health Network (REACHnet).
Why do you think the ADAPTABLE study is important?
I believe ADAPTABLE is important for three main reasons:
- Direct impact on health care—results of the ADAPTABLE study will directly impact the health care of millions of Americans and provide evidence to answer a simple question: Which dose of aspirin is more effective for preventing heart attacks and strokes in people with heart disease, a leading cause of morbidity and mortality in the U.S.? Moreover, daily aspirin therapy is a highly cost-effective clinical intervention for preventing cardiovascular disease related events.
- Demonstrate the capacity of PCORnet —In ADAPTABLE, we will be answering an important clinical question while simultaneously testing a new approach to conducting pragmatic clinical trials. We will leverage a national research infrastructure to test streamlined and innovative processes for identifying and recruiting eligible patients, obtaining informed consent electronically, and collecting data directly from participants through an online portal. ADAPTABLE also provides an opportunity to put our partnerships into practice and learn from one another. The CDRNs involved communicate and share tips, resources, and best practices. We can also learn from each other on what does not work and what challenges we encounter and possible solutions. Learnings from ADAPTABLE will ultimately be used to strengthen the PCORnet infrastructure for future large-scale trials.
- A classic example of patient-centered comparative effectiveness research (CER). In ADAPTABLE, we have a head-to-head comparison of two doses of aspirin, a medicine that people are familiar with and a design that can be used to easily conceptualize CER for both scientific and lay audiences. What makes CER different from other clinical research is that we are not using a placebo, we are studying the effectiveness, risks, and benefits of two doses of a currently available treatment. ADAPTABLE provides a relatively simple model that we can use to illustrate the potential impact of CER on health care decision-making by clinicians as well as patients.
How does clinical research benefit from patient partnerships?
Patient partners are invaluable in the research process. Their perspectives are really the most important of all involved, because clinical research relies on patients’ willingness to participate as research subjects, which requires generosity of time, information, and their bodies—these are no small things to voluntarily lend to research for the greater good of other patients and to the overall healthcare field. Patient partners ensure that scientists understand and incorporate patient preferences in the design and implementation of clinical research, which hopefully makes participating in studies seem more attractive and valuable to patients. Including patient partners in clinical research may also enhance the trustworthiness and utility of results that are then more likely to improve clinical practice and health outcomes.
In ADAPTABLE there is a team of people called the Adaptors who have coronary heart disease and are part of the research team and members of the executive and the steering committees. The Adaptors are supported by the Health eHeart Alliance and helped design the protocol, consent form, and website content. The Adaptors will provide ongoing review of study materials and will help disseminate study results to the community.
How does REACHnet collaborate with their Adaptor?
We have met with our patient partner several times in-person and on conference calls, and we correspond regularly by email. He reviewed our recruitment materials for ADAPTABLE, including: 1) an informational video shown to eligible patients on tablets, 2) a postcard given to eligible patients in clinic, and 3) a recruitment letter that will be sent by post or email. He helped ensure that the information in these materials was clear, sufficient, and concise. He also helped select attractive graphics to use on the postcard and in the video. Most importantly, our Adaptor drafted a reminder email that will be sent to patients three days after the first recruitment email if they haven’t yet enrolled. The message is written personally from him and includes a short description of his experience as a patient with CVD and reasons why he thinks it’s important for patients to join ADAPTABLE. The email will include a photo of him. We hope that this personal touch will be of interest to our target patient population and convey that this study is meant to benefit millions of patients with heart disease.
What did you and your team do to prepare for institutional review board (IRB) review and approval?
Preparing our partner IRBs for involvement in PCORnet studies and establishing good relationships are our keys to success in receiving IRB approval quickly for ADAPTABLE. Early on, we convened an IRB workgroup where we were able to establish relationships with representatives from our partner IRBs. We proactively introduced ADAPTABLE to the workgroup and learned early on what questions and concerns the IRBs had about the study. We asked: “What information do you need from us?” What are your concerns?”
One of the concerns raised was patient understanding. Due to the use of self-directed electronic consent, the IRBs wanted assurance that participants would understand the study and what is expected of them if they decided to participate. Knowing this information upfront, we provided a “walk-through,” a demonstration of how a participant would learn about the study and how their comprehension would be assessed prior to signing consent. Our site and co-principal investigators are great champions of the study and were involved in these conversations with the IRB representatives. We were able to have face-to-face discussions and readily provide information that would address concerns and questions in advance of the IRB review.
What tips or information would you recommend for a smooth and quick contract execution?
Direct communication is the primary tip I would recommend to others. Instead of addressing issues via e-mail, I set up conference calls with our partners’ legal representatives, REACHnet’s contracts manager, and myself so we could efficiently address questions from all parties in order to execute the contract quickly. Without these calls, I would have had to relay information among those involved that would potentially result in further questions and delay the timeline. I wanted to reach an agreement on the terms quickly so we could keep working on plans for implementing the study.
The ADAPTABLE study is funded through a Patient-Centered Outcomes Research Institute (PCORI) Award (Contract Number: ASP-1502-27079). ADAPTABLE is the first demonstration project to be conducted through PCORnet, the National Patient-Centered Clinical Research Network. PCORnet is a PCORI-funded initiative designed to improve the nation’s capacity to conduct comparative clinical effectiveness research. More information about PCORnet can be found here.
The post ADAPTABLE: Answering an Important Clinical Question while Testing a New Approach to Conducting Pragmatic Clinical Trials appeared first on Adaptable.
]]>Continue Reading
The post PCORnet Announces Enrollment of First Participant in ADAPTABLE Aspirin Study appeared first on Adaptable.
]]>PCORnet, the National Patient-Centered Clinical Research Network
www.pcornet.org
FOR IMMEDIATE RELEASE on Wednesday, April 27, 2016
Washington, D.C. – PCORnet, the National Patient-Centered Clinical Research Network today announced enrollment of the first patient in its ADAPTABLE (Aspirin Dosing: A Patient-centric Trial Assessing Benefits and Long-Term Effectiveness) study, a three-year pragmatic clinical trial that will compare the effectiveness of two different daily doses of aspirin widely used to prevent heart attacks and strokes in individuals living with heart disease. Pragmatic trials are designed to reflect “real-world” medical care by recruiting broad populations of patients, embedding the trial into the usual healthcare setting, and leveraging data from health systems to produce results that can be readily used to improve patient care.
“PCORnet is shifting the research paradigm through studies like ADAPTABLE by introducing a new genre of patient-centered research where patients are engaged to improve the science of clinical trial design,” said Adrian F. Hernandez, MD, MHS, director of outcomes and health services research at the Duke Clinical Research Institute (DCRI) and PCORnet’s Coordinating Center Co-Principal Investigator.
Using electronic health records (EHRs) and web-based technology, participants will self-consent, self-randomize and report data directly through an online portal. In addition, with participants’ consent, electronic health information captured during routine care will be used to identify events that will provide ADAPTABLE researchers with additional health outcomes data to inform better decision-making. Watch Dr. Hernandez’s video explaining how PCORnet and ADAPTABLE are re-thinking how clinical trials are conducted.
ADAPTABLE is funded through a research award totaling up to $18 million from the Patient-Centered Outcomes Research Institute (PCORI). DCRI is the Coordinating Center for this first demonstration study of PCORnet, an innovative initiative of PCORI involving 33 individual networks working together to make it faster, easier, and less costly to conduct clinical research by harnessing the power of large amounts of health data and patient partnerships. The study plans to enroll 20,000 patients with heart disease identified from EHRs from seven of PCORnet’s Clinical Data Research Networks (CDRNs) and 30 different health systems. The Health eHeart Alliance Patient-Powered Research Network (PPRN) supports the Adaptors, a team of people with coronary heart disease who helped design the protocol, consent form, portal content, and study materials. Together the Adaptors and Health eHeart Alliance have developed patient-centered processes to facilitate engagement.
In ADAPTABLE, the role of the patient has shifted from that of a participant to partner. “Adaptors provide more than input, they are part of the research team and members of the executive and the steering committees. Two patients who are not Adaptors will sit on the data safety monitoring board. We have not seen this type of participation in traditional clinical research. We are learning a great deal from our patient partners and along the way we are transforming how clinical research is performed,” said Lisa G Berdan, Director, DCRI Global Cardiovascular Outcomes Trials.
Jacqueline Alikhaani, an Adaptor, offers this perspective, a view that is shared by her fellow Adaptors, “What we are doing in ADAPTABLE is landmark work. For the first time ever we are looking to improve patient-centered care with patients at the table, not just on the table. Research is necessary to determine if and how changes can best be made. This is why I volunteered to help design and implement the ADAPTABLE aspirin study.”
Heart disease is the leading cause of death for both men and women. About 610,000 people die of heart disease in the United States every year. It accounts for one in every four deaths and is a disease that places enormous burdens not only on patients and their families but on the nation and its healthcare system as a whole.
ADAPTABLE Steering Committee Co- Chairperson, Russell Rothman, MD, MPP, Principal Investigator of the Mid-South CDRN said, “Typically when a patient is diagnosed with heart disease they are prescribed baby or low-dose aspirin (81 mg) or regular strength (325mg). However, we actually don’t know the optimal dose of aspirin for balancing benefits in preventing a future heart attack or stroke against its risk of bleeding. Results of the ADAPTABLE study will provide doctors with a better understanding of which dose of aspirin is best for which patient so that we can improve healthcare delivery and patient outcomes.” Watch Dr. Rothman’s video to learn about the significance of ADAPTABLE and novel aspects of its design as a pragmatic clinical trial.
ADAPTABLE will help patients and health care providers determine the right dose to use in coronary artery disease. In addition, it will provide a new framework for how clinical trials can be delivered. For more information, visit www.theaspirinstudy.net or check out the patient portal at www.adaptablepatient.com.
###
About PCORnet
PCORnet, the National Patient-Centered Clinical Research Network, is an innovative initiative of the Patient-Centered Outcomes Research Institute (PCORI). The goal of PCORnet is to improve the nation’s capacity to conduct comparative clinical effectiveness research efficiently by creating a large, highly representative network for conducting clinical outcomes research that directly involves patients in the development and execution of the research. More information is available at www.pcornet.org.
About PCORI
The Patient-Centered Outcomes Research Institute (PCORI) is an independent, nonprofit organization authorized by Congress in 2010. Its mission is to fund research that will provide patients, their caregivers, and clinicians with the evidence-based information needed to make better-informed healthcare decisions. PCORI is committed to continually seeking input from a broad range of stakeholders to guide its work. More information is available at www.pcori.org.
Media Contact
Mark Slagle
(919) 668-8031
PCORnet Coordinating Center
The post PCORnet Announces Enrollment of First Participant in ADAPTABLE Aspirin Study appeared first on Adaptable.
]]>The post ADAPTABLE Presented to the American College of Cardiology Board of Governors appeared first on Adaptable.
]]>
The post ADAPTABLE Presented to the American College of Cardiology Board of Governors appeared first on Adaptable.
]]>